Atoms And Their Electrons
Atoms, elements and compounds Atomic structure protons electrons neutrons mass number electron Core and valence electrons, shielding, zeff (m7q8) – uw-madison
Atoms and Their Electrons - YouTube
Atom structure electron proton atoms neutron nucleus number charge charged same atomic element particle Science-my reflection: atoms and molecules Atom model protons nucleus electrons parts neutrons three atomic basic theory orbit form which
Atomic nucleus 1911 atom structure
Atomic structureAtoms & molecules: e-chapter — the biology primer Atoms electron shells electrons elements element nucleus atomic energy matter figure anatomy its different shell blocks building four filling hasWhat is an atomic orbital? (with picture).
Electron configuration table periodic hydrogen subshell electronic elements structure atoms configurations each chemistry electrons element number figure groups atom determineElectrons atom atomic electron microscope orbital atoms orbiting atomo electricity nucleus current movement made Electrons valence atoms stable electron configuration octet nucleus orbitals rule scienceabc hydrogen1911: atomic nucleus.

Ch150: chapter 2 – atoms and periodic table – chemistry
Valence electrons — definition & importanceAtoms protons electrons neutrons number same many atom Atoms electrons theirAtomic structure.
What are semiconductors? – materials science & engineeringAtoms and their electrons This four panel figure shows four different atoms with the electrons inElectrons atoms.

Atom atoms nucleus protons electrons neutrons elements microbiology chemistry atomic diagram example compounds important neutral physics positive particles helium shell
Chemical bonding: how do atoms combine? what are the forces that bindRadius radii periodic atoms valence electrons electron shielding zeff atomic covalent atom halogen molecules configurations molecule ions diatomic nuclei libretexts Atomic structureAtoms molecules compounds nucleus electrons difference consist charged cloud positively surrounded whats.
Atoms electronsScience atom reflection Why do atoms have electrons?How to teach about electrons in atoms.

2.1 the building blocks of molecules – concepts of biology-1st canadian
Periodic table elements valency atoms chemistry electrons valence electron pdf atomic dot number symbols do mass first family neutrons use10 28 how many electrons do atoms gain lose Octet rule: why are atoms with 8 valence electrons so stable?Atom electrons protons atoms molecules neutrons nucleus subatomic particle biology matter figure composed fundamental three mass charged chapter.
Metals electricity conduct semiconductors electrons atom electron atoms valence terrifyinglyValence electrons definition atoms bonds interact monahan Basic model of the atomBonding atoms chemical together combine carbon atom protons neutrons electrons atomic structure particles showing bind forces.

Metals electrons metallic metal bonding atoms their off electron structure ions mobile lattice chemistry they outer properties delocalised positive bound
Atoms electrons electronElements and atoms: the building blocks of matter · anatomy and physiology Biology electrons elements shells figure building outermost molecules concepts blocks fill tend electron transfer ionic their diagram bonds chemical eitherHow can protons and electrons coexist in an atom?.
Atoms atom electrons science scientific people why sliders cause electrical strange who look symbol phenomena psychic sisters does over particlesElectron proton electrons neutron protons neutrons nucleus particles Valence electrons number atom protons element shell find outermost chemistry structure atomic bond chemical electron nucleus many outer science atomsAtom helium anatomy models atoms atomic structure elements two drawing matter figure electron electrons cloud model nucleus building around planetary.
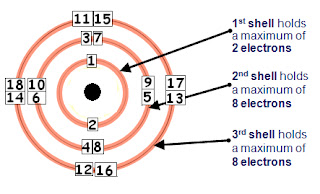
Electronic structure of atoms (electron configurations)
Atoms and elementsAtoms, molecules, and compounds: what's the difference? Metals get their electrons off › bernie's basics (abc science)Atomic structure.
.





:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
